Category: Acids
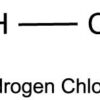
Hydrogen Chloride (HCl)
Hydrogen chloride (HCl) is an inorganic compound of two elements: hydrogen (atomic number 1) and chlorine (atomic number 17). Hydrogen chloride is a colourless to yellowish, corrosive, nonflammable gas at room temperature. The gas is heavier than air and has a strong irritating odor. It is readily soluble in water and forms an acidic solution. Its solution in water is called hydrochloric acid. Hydrochloric acid is a very strong acid. It hydrolyzes in water into H+ (H3O+) and Cl﹣.