Category: Solution Calculations
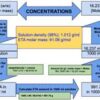
Molarity of 25% (w/w) Hydrochloric Acid (HCl)
The molarity of 25% (w/w) hydrochloric acid is 7.68 M. The 25% (w/w) hydrochloric acid contains 25g of HCl per 100g of solution. Since molarity is the number of moles of a substance present in a liter of solution, one must first calculate how much HCl is present in 1L of 25% hydrochloric acid solution. Once we know the amount of HCl present in 1L of solution, we can calculate the molarity of the solution by dividing the HCl amount by the HCl molecular weight.
Preparation of 1M Hydrochloric Acid From Concentrated Stock Solution (37%, w/w)
A 37% (w/w) hydrochloric acid can be purchased from several suppliers. It is a clear colorless liquid and can be diluted to prepare solutions of known concentrations. Remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1M solution is also a 1N solution. Here we describe a procedure to prepare 1M hydrochloric acid solution by diluting a 37% (w/w) concentrated hydrochloric acid solution.
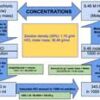
Molarity of 37% (w/w) Hydrochloric Acid (HCl)
A 37% (w/w) concentrated Hydrochloric acid is a clear colorless liquid. It is an aqueous solution of hydrogen chlori (HCl). The 100 g of 37% (w/w) Hydrochloric acid contains 37 g of HCl. To calculate the molarity, one must first calculate how much HCl is present in 1 L of 37% Hydrochloric acid solution. Once we know the amount of HCl present in 1 L solution, we can calculate the molarity of the solution by dividing the HCl amount by the HCl molecular weight.
![Bromobenzene [C6H5Br] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/01/fi-bromobenzene-molecular-weight-calculation-100x65.jpg)