Tag: hcl
Preparation of 1M Hydrochloric Acid From Concentrated Stock Solution (37%, w/w)
A 37% (w/w) hydrochloric acid can be purchased from several suppliers. It is a clear colorless liquid and can be diluted to prepare solutions of known concentrations. Remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1M solution is also a 1N solution. Here we describe a procedure to prepare 1M hydrochloric acid solution by diluting a 37% (w/w) concentrated hydrochloric acid solution.
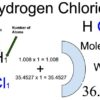
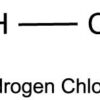
Hydrogen Chloride (HCl)
Hydrogen chloride (HCl) is an inorganic compound of two elements: hydrogen (atomic number 1) and chlorine (atomic number 17). Hydrogen chloride is a colourless to yellowish, corrosive, nonflammable gas at room temperature. The gas is heavier than air and has a strong irritating odor. It is readily soluble in water and forms an acidic solution. Its solution in water is called hydrochloric acid. Hydrochloric acid is a very strong acid. It hydrolyzes in water into H+ (H3O+) and Cl﹣.
Molarity of 37% (w/w) Hydrochloric Acid (HCl)
A 37% (w/w) concentrated Hydrochloric acid is a clear colorless liquid. It is an aqueous solution of hydrogen chlori (HCl). The 100 g of 37% (w/w) Hydrochloric acid contains 37 g of HCl. To calculate the molarity, one must first calculate how much HCl is present in 1 L of 37% Hydrochloric acid solution. Once we know the amount of HCl present in 1 L solution, we can calculate the molarity of the solution by dividing the HCl amount by the HCl molecular weight.