Category: Laboratory Calculations
N-Nitroso-N-Methylurea (NMU) [C2H5N3O2] Molecular Weight Calculation
N-Nitroso-N-methylurea (NMU) (C2H5N3O2) is an organic compound of four elements: Carbon, Hydrogen, Nitrogen, and Oxygen. The molecular weight of N-Nitroso-N-methylurea (NMU) is 103.08042 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements.
Disodium Hydrogen Arsenate [Na2HAsO4] Molecular Weight Calculation
Disodium hydrogen arsenate [Na2HAsO4] is an inorganic compound of four elements: Sodium (Na), Hydrogen, Arsenic (As), and Oxygen (O). The molecular weight of Disodium hydrogen arsenate is 185.90673356 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements.
Sodium Dihydrogen Arsenate Monohydrate [NaH2AsO4.H2O] Molecular Weight Calculation
Sodium dihydrogen arsenate [NaH2AsO4] is an inorganic compound of four elements: Sodium (Na), Hydrogen, Arsenic (As), and Oxygen (O). The monohydrate form of Sodium dihydrogen arsenate [NaH2AsO4.H2O] also contains one water (H2O) molecule. The molecular weight of Sodium dihydrogen arsenate monohydrate is 181.94036428 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements and water molecule.
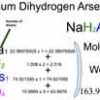
Sodium Dihydrogen Arsenate (NaH2AsO4) Molecular Weight Calculation
Sodium dihydrogen arsenate [NaH2AsO4] is an inorganic compound of four elements: Sodium (Na), Hydrogen, Arsenic (As), and Oxygen (O). The molecular weight of Sodium dihydrogen arsenate is 163.92496428 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements.
Disodium Methylarsonate [CH3AsNa2O3] Molecular Weight Calculation
Disodium methylarsonate [CH3AsNa2O3] is an organic compound of five elements: Carbon (C), Hydrogen (H), Arsenic (As), Sodium (Na), and Oxygen (O). The molecular weight of Disodium methylarsonate is 183.93403356 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Carbon, Hydrogen, Arsenic, Sodium, and Oxygen.
Phaunouxite [Ca3(AsO4)2.11H2O] Molecular Weight Calculation
Calcium arsenate [Ca3(AsO4)2] is an inorganic compound of three elements: Calcium (Ca), Arsenic (As), and Oxygen (O). The undecahydrate form of Calcium arsenate, Phaunouxite [Ca3(AsO4)2.11H2O], also contains eleven water (H2O) molecules. The molecular weight of Phaunouxite is 596.24179 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements and water molecules.
Calcium Arsenate [Ca3(AsO4)2] Molecular Weight Calculation
Calcium arsenate [Ca3(AsO4)2] is an inorganic compound of three elements: Calcium (Ca), Arsenic (As), and Oxygen (O). The molecular weight of Calcium arsenate is 398.07239 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Calcium, Arsenic, and Oxygen.
Methylarsonic Acid [CH3AsO(OH)2] Molecular Weight Calculation
Methylarsonic acid [CH3AsO(OH)2] is an organic compound of four elements: Carbon (C), Hydrogen (H), Arsenic (As), and Oxygen (O). The molecular weight of Methylarsonic acid is 139.970495 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements.
Roxarsone [C6AsNH6O6] Molecular Weight Calculation
Roxarsone [C6AsNH6O6] is an organic compound of five elements: Carbon (C), Arsenic (As), Nitrogen (N), Hydrogen (H), and Oxygen (O). The molecular weight of Roxarsone is 263.036935 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Carbon, Arsenic, Nitrogen, Hydrogen, and Oxygen.
![N-Nitroso-N-Methylurea (NMU) [C2H5N3O2] Molecular Weight Calculation N-Nitroso-N-Methylurea (NMU) [C2H5N3O2] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2024/01/fi-n-nitroso-n-methylurea-nmu-molecular-weight-calculation-100x100.jpg)
![PD98059 [C16H13NO3] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/07/fi-pd98059-molecular-weight-calculation-100x95.jpg)
![Disodium Hydrogen Arsenate [Na2HAsO4] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/06/fi-disodium-hydrogen-arsenate-molecular-weight-calculation-100x100.jpg)
![Sodium Dihydrogen Arsenate Monohydrate [NaH2AsO4.H2O] Molecular Weight Calculation Sodium Dihydrogen Arsenate Monohydrate [NaH2AsO4.H2O] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-sodium-dihydrogen-arsenate-monohydrate-molecular-weight-calculation-100x100.jpg)
![Disodium Methylarsonate [CH3AsNa2O3] Molecular Weight Calculation Disodium Methylarsonate [CH3AsNa2O3] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-disodium-methylarsonate-molecular-weight-calculation-100x100.jpg)
![Phaunouxite [Ca3(AsO4)2.11H2O] Molecular Weight Calculation Phaunouxite [Ca3(AsO4)2.11H2O] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-phaunouxite-molecular-weight-calculation-100x100.jpg)
![Rauenthalite [Ca3(AsO4)2.10H2O] Molecular Weight Calculation Rauenthalite [Ca3(AsO4)2.10H2O] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-rauenthalite-molecular-weight-calculation-100x100.jpg)
![Calcium Arsenate [Ca3(AsO4)2] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-arsenous-acid-molecular-weight-calculation-1-100x96.jpg)
![Sodium Arsenate [Na3AsO4] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-sodium-arsenate-molecular-weight-calculation-100x91.jpg)
![Sodium Orthoarsenite [Na3AsO3] Molecular Weight Calculation Sodium Orthoarsenite [Na3AsO3] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-sodium-orthoarsenite-molecular-weight-calculation-100x91.jpg)
![Arsenic(III) Telluride [As2Te3] Molecular Weight Calculation Arsenic(III) Telluride [As2Te3] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-arsenic-iii-tellurid-molecular-weight-calculation-100x88.jpg)
![Methylarsonic Acid [CH3AsO(OH)2] Molecular Weight Calculation Methylarsonic Acid [CH3AsO(OH)2] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/03/fi-methylarsonic-acid-molecular-weight-calculation-100x100.jpg)
![Roxarsone [C6AsNH6O6] Molecular Weight Calculation Roxarsone [C6AsNH6O6] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-roxarsone-molecular-weight-calculation-100x100.jpg)
![Trimethylarsine [(CH3)3As] Molecular Weight Calculation Trimethylarsine [(CH3)3As] Molecular Weight Calculation](https://www.laboratorynotes.com/wp-content/uploads/2023/05/fi-trimethylarsine-molecular-weight-calculation-100x95.jpg)