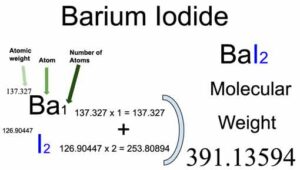
The molecular weight of Barium iodide [BaI2] is 391.13594.
 Barium iodide [BaI2] is an inorganic compound of two elements: Barium and Iodine. The molecular weight of Barium iodide is 391.13594 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Barium and Iodine.
Barium iodide [BaI2] is an inorganic compound of two elements: Barium and Iodine. The molecular weight of Barium iodide is 391.13594 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Barium and Iodine.
CALCULATION PROCEDURE: Barium iodide [BaI2] Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in a Barium iodide molecule.
From the chemical formula, you will know different atoms and their number in a Barium iodide molecule. The chemical formula of Barium iodide is BaI2. From the chemical formula, you can find that one molecule of Barium iodide has one Barium (Ba) atom and two Iodine (I) atoms.
Step 2: Find out the atomic weights of each atom (from the periodic table).
Atomic weight of Barium (Ba): 137.327 (Ref: Pubchem-56, Ciaaw-barium)
Atomic weight of Iodine (I): 126.90447 (Ref: Jlab-ele053, Ciaaw-iodine, Nist-53)
Step 3: Calculate the molecular weight of Barium iodide by adding the total weight of all atoms.
Number of Barium atoms in Barium iodide: 1
Atomic weight of Barium: 137.327
Total weight of Barium atoms in Barium iodide: 137.327 x 1 = 137.327
Number of Iodine atoms in Barium iodide: 2
Atomic weight of Iodine: 126.90447
Total weight of Iodine atoms in Barium iodide: 126.90447 x 2 = 253.80894
Step 4: Calculate the molecular weight of Barium iodide by adding up the total weight of all atoms.
Molecular weight of Barium iodide (BaI2): 137.327 (Barium) + 253.80894 (Iodine) = 391.13594
So the molecular weight of Barium iodide is 391.13594.
Barium iodide [BaI2] Molecular Weight Calculation
| Molecular weight of Barium iodide (BaI2) | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| Barium (Ba) | 1 | 137.327 | 137.327 |
| Iodine (I) | 2 | 126.90447 | 253.80894 |
| Molecular weight of Barium iodide (BaI2): | 391.13594 | ||
REFERENCES:
- Pubchem-56: https://pubchem.ncbi.nlm.nih.gov/element/56
- Ciaaw-barium: https://www.ciaaw.org/barium.htm
- Jlab-ele056: https://education.jlab.org/itselemental/ele056.html
- Jlab-ele053: https://education.jlab.org/itselemental/ele053.html
- Ciaaw-iodine: https://www.ciaaw.org/iodine.htm
- Nist-53: https://physics.nist.gov/cgi-bin/Elements/elInfo.pl?element=53