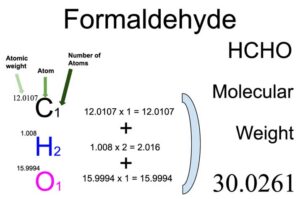
The molecular weight of Formaldehyde (HCHO) is 30.0261.
 To calculate molecular weight of any compound, the first step is to know the constituent atoms and their number in that particular compound. Then calculate the total weight of each atom by multiplying its atomic weight by its number. The sum of total weight of all constituent atoms will be the molecular weight of the compound. Note that the value of atomic weight may differ slightly from different sources.
To calculate molecular weight of any compound, the first step is to know the constituent atoms and their number in that particular compound. Then calculate the total weight of each atom by multiplying its atomic weight by its number. The sum of total weight of all constituent atoms will be the molecular weight of the compound. Note that the value of atomic weight may differ slightly from different sources.
CALCULATION PROCEDURE: Formaldehyde (HCHO) Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in a Formaldehyde molecule.
From the chemical formula, you will know different atoms and their number in a Formaldehyde molecule. Chemical formula of Formaldehyde is CH2O. From the chemical formula of Formaldehyde, you can find that one molecule of Formaldehyde has one Carbon (C) atom, two Hydrogen (H) atoms and one Oxygen (O) atom.
Step 2: Find out atomic weights of each atom (from periodic table).
Atomic weight of Carbon (C): 12.0107 (Ref: Jlab-ele006)
Atomic weight of Hydrogen (H) : 1.008 (Ref: Lanl-1)
Atomic weight of Oxygen (O) : 15.9994 (Ref: Jlab-ele008)
Step 3: Calculate the total weight of each atom present in a Formaldehyde molecule by multiplying its atomic weight by its number.
Number of Carbon atoms in Formaldehyde: 1
Atomic weight of Carbon: 12.0107
Total weight of Carbon atoms in Formaldehyde: 12.0107 x 1 = 12.0107
Number of Hydrogen atoms in Formaldehyde: 2
Atomic weight of Hydrogen: 1.008
Total weight of Hydrogen atoms in Formaldehyde: 1.008 x 2 = 2.016
Number of Oxygen atoms in Formaldehyde: 1
Atomic weight of Oxygen: 15.9994
Total weight of Oxygen atoms in Formaldehyde: 15.9994 x 1 = 15.9994
Step 4: Calculate the molecular weight of Formaldehyde by adding up the total weight of all atoms.
Molecular weight of Formaldehyde: 12.0107 (Carbon) + 2.016 (Hydrogen) + 15.9994 (Oxygen) = 30.0261
So the molecular weight of Formaldehyde is 30.0261.
Formaldehyde (HCHO) Molecular Weight Calculation
| Molecular weight of Formaldehyde | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| Carbon (C) | 1 | 12.0107 | 12.0107 |
| Hydrogen (H) | 2 | 1.008 | 2.016 |
| Oxygen (O) | 1 | 15.9994 | 15.9994 |
| Molecular weight of Formaldehyde: | 30.0261 | ||
REFERENCES:
- Lanl-1: https://periodic.lanl.gov/1.shtml
- Pubchem-Hydrogen : https://pubchem.ncbi.nlm.nih.gov/element/Hydrogen
- Jlab-ele006; https://education.jlab.org/itselemental/ele006.html
- Pubchem-6: https://pubchem.ncbi.nlm.nih.gov/element/6
- Jlab-ele008: https://education.jlab.org/itselemental/ele008.html
- Pubchem-8: https://pubchem.ncbi.nlm.nih.gov/element/8