OVERVIEW
- A 48% (w/w) Hydrofluoric acid (HF) is a clear colorless aqueous solution of Hydrogen fluoride (HF) gas. It can be purchased from many commercial suppliers.
- % refers to solution concentration in percentage and “(w/w)” refers to solute and solution amount given in grams (i.e., percentage by weight). This means a 48% (w/w) Hydrofluoric acid contains 48 g of Hydrogen fluoride (HF) per 100 g of solution.
- The density of 48% hydrofluoric acid is 1.15 g/ml at 25° C. This means the weight of the 1 ml of hydrofluoric acid is 1.15 g at 25°C.
- Molarity refers to the number of moles of the solute per litre of solution. To calculate the Molarity of 48% (w/w) Hydrofluoric Acid, you need to know how many moles of HF are present in 1 liter of solution.
- In simple words, 1 mole is equal to the molecular weight of the substance. For example, 1 mole of Hydrogen fluoride is equal to 20.01 g of Hydrogen fluoride (Molecular Weight: 20.01).
- To calculate the molarity, one must first calculate how much Hydrogen fluoride is present in 1 L of 48% Hydrofluoric acid. Once we know the amount of HF present in 1 L solution, we can calculate the molarity of the solution by dividing the HF amount by the molecular weight.
- Calculator: Converting Percentage by Mass to Molarity
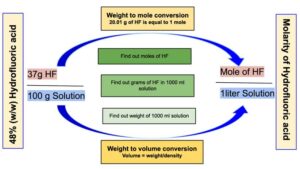
Calculation procedure
| Known values | |
| Molar mass of Hydrogen fluoride | 20.01 g/mole |
| Concentration of Hydrofluoric acid | 48% (% by mass, w/w) |
| Density of 37% Hydrofluoric acid | 1.15 g/ml |
Step 1: Calculate the volume of 100 grams of Hydrofluoric acid.
Formula:
Density =
OR
Volume =
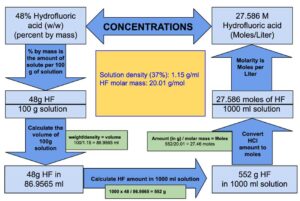
The volume of 100 g of Hydrofluoric acid : = 86.9565 ml
Note: 48% (w/w) Hydrofluoric acid means that 100 g of Hydrofluoric acid contains 48 g of HF.
The volume of 100 g of Hydrofluoric acid is 86.9565 ml which means 48 g of HF is present in 86.9565 ml of Hydrofluoric acid.
Step 2: Calculate how many grams of HF is present in 1000 ml of Hydrofluoric acid.
86.9565 ml of Hydrofluoric acid contains = 48 grams of HF
1 ml of Hydrofluoric acid will contain = grams of HF
1000 ml of Hydrofluoric acid will contain = = 552 grams of HF
1000 ml of Hydrofluoric acid will contain 552 grams of HF.
Step 3: Calculate the number of moles of HF in 552 grams of HF.
20.01 g of HF is equal to 1 mole.
1 g of HF will be equal to moles.
552 grams of Hydrogen fluoride will be equal to = = 27.46 moles
Therefore, we can say that 1 liter of Hydrofluoric acid contains 27.46 moles of HF or in other words molarity of 48% (w/w) Hydrofluoric acid is equal to 27.46 M.
Calculator – Calculate the molarity of concentrated Hydrofluoric acid (HF)
Hydrogen Fluoride (HF) Molecular weight: 20.01 g/mole
4 thoughts on “Molarity of 48% (w/w) Hydrofluoric Acid (HF)”