OVERVIEW
- DNA sample is mixed with DNA loading dye prior to loading onto the wells of agarose gel.
- A DNA loading dye must contain at least one tracking dyes (orange G, bromophenol blue, xylene cyanol FF or bromocresol green) and a high density reagent (glycerol, sucrose or Ficoll 400).
- A buffering agent can also be added to maintain the pH of DNA loading dye.
- DNA loading dye supplemented with a buffer, usually Tris buffer, is called DNA loading buffer.
- Tracking dyes help to track the progression of gel electrophoresis and sample loading process in the well.
- The high density reagent (glycerol, sucrose or Ficoll 400) is added to increase the density of the sample.
- Due to high density, the sample settles at the bottom of the well. It also helps a DNA sample to be confined in the well without diffusing out.
- Two tracking dyes, orange G and xylene cyanol FF, containing DNA loading buffers are very common for DNA gel electrophoresis.
- Orange G (C16H10N2Na2O7S2; Molar mass – 452.369339 gram/moleRef) is one of the most commonly used electrophoresis tracking dyes.
- Orange G, a major component in the Papanicolaou stain, is an azo dye used primarily as a histological stain.
- Orange G is available commercially as an orange crystals or powder (Color Index Number: 16230) which is highly soluble in water (solubility in water is ~ 80 mg/ml).
- Orange G usually comes as a disodium salt. The color of aqueous solution of Orange G is pH dependent.
- Orange G solution appears yellow/orange at neutral or acidic pH and red at pH greater than 9.
- Xylene cyanol FF (C25H27N2NaO6S2 ; Molar mass – 538.61 gram/mole) is available commercially as a dark green crystalline powder.
- Both tracking dyes, Orange G and Xylene cyanol FF, are soluble in water (solubility in water is ~ 1 mg/ml) and carry net negative charge at neutral or slightly basic pH (the pH of the electrophoresis buffer).
- The percentage of agarose gel influences the moving position of orange G and xylene cyanol FF in the gel. The orange G and xylene cyanol FF co-migrates with ~50 bp and ~4000 bp DNA fragments in 1% agarose gel respectively.
- Ficoll 400 is a high molecular weight, neutral, hydrophilic, polysaccharide. It is added to provide high density to the sample.
- Tris (Molecular Formula : C4H11NO3; Molar mass – 121.13504 gram/mole) acts as a buffering agent and maintains the pH of solution. The buffering pH range of Tris is 7.0 to 9.2 that coincides with the physiological pH of most living organisms.
- The pH value of Tris buffer is temperature and concentration dependent. For Tris buffers, pH increases about 0.025 – 0.03 unit per degree centigrade decrease in temperature, and decreases 0.03 – 0.05 unit per ten-fold dilution.
- A 6X DNA loading buffer containing orange G, Xylene cyanol FF and Ficoll 400 appears dark green in color.
- A 6X DNA loading buffer can have a wide range of Xylene cyanol [0.03% to 0.50% (w/v)] and orange G concentration [orange G concentration – 0.25 to 0.40% (w/v)]. High concentration of dye provides very good contrast colour, which is easy to monitor upon electrophoresis progression. However, dye can mask the co-migrating DNA fragments.
- The visibility of co-migrating DNA fragments decreases with increasing dye concentration, causing interference in the analysis of co-migrating DNA bands (e.g., densitometric analysis).
- Low concentration of dye is preferred when a DNA sample is expected to contain co-migrating DNA fragment(s). However, low concentration of dye causes a compromise in the visibility of migrating dye bands, which sometimes disappear after a long electrophoresis run.
REQUIREMENTS
Reagents and solutions
Orange G
Xylene cyanol FF
Ficoll 400 (Suppliers list)
1M Tris.Cl, pH 7.6 at 25°C
Deionized / Milli-Q water
Equipments and disposables
15-ml screw-cap graduated polypropylene centrifuge tube
Centrifuge (for 15 ml tube)
Tube rotator (optional)
Vortex Mixer (optional)
COMPOSITION
Composition of 6X DNA loading dye
0.40% (w/v) orange G
0.25% (w/v) xylene cyanol FF
15% (w/v) Ficoll 400
10 mM Tris.Cl
Composition of 1X DNA loading dye
0.067% (w/v) Orange G
0.042% (w/v) xylene cyanol FF
2.5% (w/v) Ficoll 400
1.67 mM Tris.Cl, pH 7.6
OBJECTIVE
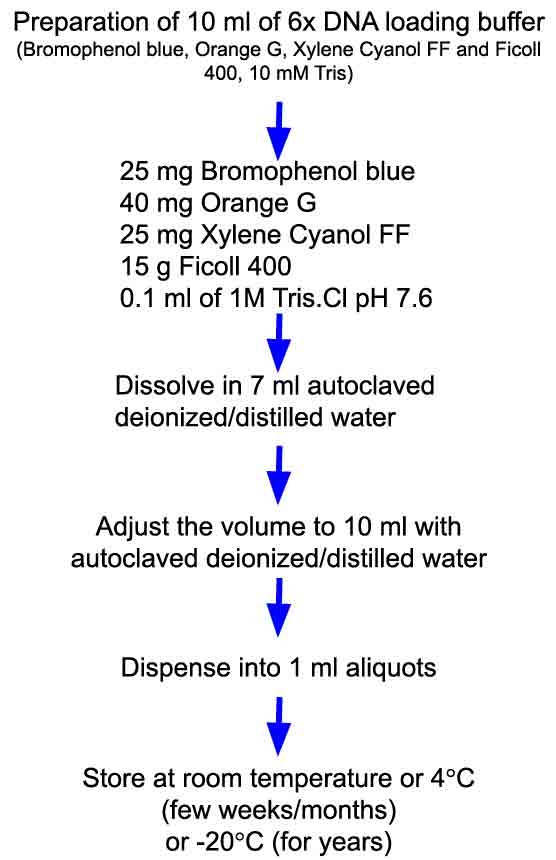
Preparation of 10 ml of 6X DNA loading dye containing orange G, xylene cyanol FF, Ficoll 400, Tris.Cl, pH 7.6
PROCEDURE
Use appropriate personal protective equipment (lab coat, gloves, goggles, etc) for your safety and follow the guidelines of your institute.
Step 1: To prepare 10 ml of 6X DNA loading dye, weigh out 40 mg orange G, 25 mg xylene cyanol FF and 1.5 g Ficoll 400. Transfer it to a 15-mL screw-capped graduated tube. Add 7 ml deionized / Milli-Q water and 0.1 ml of 1M Tris.Cl, pH 7.6. Dissolve all ingredients by inverting the tube number of times or using a rotator/vortex mixer. You can heat the solution to 60°C (in water bath) to dissolve ingredients.
Tip:
We recommend using a disposable nuclease-free 15-mL screw-capped graduated tube. It allows you to complete the whole process in a single tube without transferring liquid to the measuring cylinder. If you use a beaker or conical flask, you need to transfer the content to a measuring cylinder to adjust the volume to 10 ml. Transferring solution may not be convenient as the solution will be viscous and contains dye.
Precautions:
1. Do not dissolve in 10 ml of deionized / Milli-Q water. In most cases, solution volume increases when a large amount of solutes dissolve in a solvent.
2. Use nuclease-free, autoclaved deionized / Milli-Q water and glasswares.
Step 2: Adjust the volume to 10 ml with deionized / Milli-Q water. Mix it again.
Note: The solution will appear dark green in color with no undissolved particles. If there are any undissolved particles in the solution, remove them by centrifuging the tube at 4000 – 5000 rpm for 10 min at room temperature.
Storage
Store the solution at room temperature or 4°C or -20°C.
Tip: It is recommended to store the solution in small aliquots (1 ml).
Applications
This solution is used for loading DNA samples onto non-denaturing gels.
| Follow the table to prepare 6X agarose gel loading dye of various volumes (5 ml, 10 ml, 25 ml, 100). | |||||
| Reagents / Volume | 5 ml | 10 ml | 25 ml | 50 ml | 100 ml |
| Orange G | 20 mg | 40 mg | 100 mg | 200 mg | 400 mg |
| Xylene cyanol FF | 12.5 mg | 25 mg | 62.5 mg | 125 mg | 250 mg |
| Ficoll 400 | 0.75 g | 1.5 g | 3.75 g | 7.5 g | 15 g |
| 1 M Tris.Cl, pH 7.6 | 0.05 ml | 0.1 ml | 0.25 ml | 0.5 ml | 1 ml |
| Water | Adjust the final volume to 5 ml | Adjust the final volume to 10 ml | Adjust the final volume to 25 ml | Adjust the final volume to 50 ml | Adjust the final volume to 100 ml |
CALCULATOR
Use Calculator to calculate the amount of different components of 6X DNA loading dye.
Volume of the 6X DNA loading dye: ml
(Change the volume of the solution)
Amount of Orange G: mg
Amount of Xylene Cyanol FF: mg
Amount of Ficoll 400: g
Amount of 1 M Tris (pH 7.6): ml
1 thought on “Preparation of 6X DNA Loading Buffer (Orange G, Xylene Cyanol FF and Ficoll 400)”