The molecular weight of Silver nitrate [AgNO3] is 169.87314.
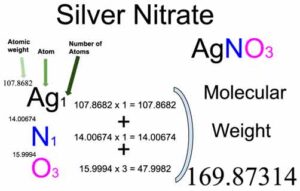 Silver nitrate [AgNO3] is an inorganic compound of three elements: Silver, Nitrogen, and Oxygen. The molecular weight of Silver nitrate is 169.87314 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Silver, Nitrogen, and oxygen.
Silver nitrate [AgNO3] is an inorganic compound of three elements: Silver, Nitrogen, and Oxygen. The molecular weight of Silver nitrate is 169.87314 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Silver, Nitrogen, and oxygen.
CALCULATION PROCEDURE: Silver Nitrate [AgNO3] Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in a Silver nitrate molecule.
From the chemical formula, you will know different atoms and their number in a Silver nitrate molecule. The chemical formula of Silver nitrate is AgNO3. From the chemical formula, you can find that one molecule of Silver nitrate has one Silver (Ag) atom, one Nitrogen (N) atom, and three Oxygen (O) atoms.
Step 2: Find out the atomic weights of each atom (from the periodic table).
Atomic weight of Silver (Ag): 107.8682 (Ref: Jlab-ele047, ciaaw-silver)
Atomic weight of Nitrogen (N): 14.00674 (Ref: Jlab-ele007)
Atomic weight of Oxygen (O): 15.9994 (Ref: Jlab-ele008)
Step 3: Calculate the molecular weight of Silver nitrate by adding the total weight of all atoms.
Number of Silver (Ag) atoms in Silver nitrate: 1
Atomic weight of Silver: 107.8682
Total weight of Silver atoms in Silver nitrate: 107.8682 x 1 = 107.8682
Number of Nitrogen (N) atoms in Silver nitrate: 1
Atomic weight of Nitrogen: 14.00674
Total weight of Nitrogen atoms in Silver nitrate: 14.00674 x 1 = 14.00674
Number of Oxygen (O) atoms in Silver nitrate: 3
Atomic weight of Oxygen: 15.9994
Total weight of Oxygen atoms in Silver nitrate: 15.9994 x 3 = 47.9982
Step 4: Calculate the molecular weight of Silver nitrate by adding up the total weight of all atoms.
Molecular weight of Silver nitrate (AgNO3): 107.8682 (Silver) + 14.00674 (Nitrogen) + 47.9982 (Oxygen) = 169.87314
So the molecular weight of Silver nitrate is 169.87314.
Silver Nitrate [AgNO3] Molecular Weight Calculation
| Molecular weight of Silver nitrate (AgNO3) | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| Silver (Ag) | 1 | 107.8682 | 107.8682 |
| Nitrogen (N) | 1 | 14.00674 | 14.00674 |
| Oxygen (O) | 3 | 15.9994 | 47.9982 |
| Molecular weight of Silver nitrate (AgNO3): | 169.87314 | ||
REFERENCES:
- Jlab-ele047: https://education.jlab.org/itselemental/ele047.html
- ciaaw-silver: https://www.ciaaw.org/silver.htm
- Jlab-ele007: https://education.jlab.org/itselemental/ele007.html
- Jlab-ele008: https://education.jlab.org/itselemental/ele008.html
See also:
Article authenticity Index: ***
* (Newly added)
***** (Revised 4 times)
********** (Revised 9 times)
# Please let us know if you find any mistakes. Your comments are welcome in the comment box.