OVERVIEW
Hydrochloric acid is an aqueous solution of hydrogen chloride gas. The density of 37% (w/w) hydrochloric acid solution is 1.2 g/ml. The molar mass of hydrogen chloride is 36.46 g/mol. 37% (w/w) hydrochloric acid means 37 g hydrogen chloride is present in 100 g solution
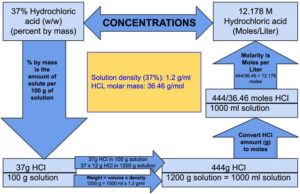
Step 1: Since molarity refers to number of moles in 1000 ml solution, calculate the weight of 1000 ml hydrochloric acid using the following formula:
Weight = volume x density
Weight = 1000 x 1.2 = 1200
Weight of 1000 ml hydrochloric acid is 1200 g.
Step 2: Calculate how much hydrogen chloride is in 1200 g of hydrochloric acid.
100 g hydrochloric acid contains: 37 g of hydrogen chloride
1 g hydrochloric acid will contain: 37/ 100 g of hydrogen chloride
1200 g hydrochloric acid will contain: 1200 x 37/ 100 = 444 g of hydrogen chloride
Note: Since the volume of 1200 g of hydrochloric acid solution is 1000 ml, we can say that 444 g of hydrochloride is present in 1000 ml of hydrochloric acid solution.
Step 3: Calculate number of moles in 444 g of hydrogen chloride using following formula
Number of moles: weight (in grams)/molar mass (or molecular weight)
Number of moles in 444 g of hydrogen chloride = 444 /36.46 = 12.178 moles.
It means 12.178 moles of hydrogen chloride is present in 1000 ml of hydrochloric acid solution.
Molarity refers to the number of moles present in 1000 ml of solution, therefore the molarity (moles/liter) of 37% hydrochloric acid solution is 12.178 M.
5 thoughts on “How to Calculate Molarity of 37% (w/w) Hydrochloric acid (HCl), Alternative Method”