The molarity of 30% (w/w) Hydrochloric Acid is 9.46 M.
OVERVIEW
A 30% (w/w) Hydrochloric Acid is a clear colorless aqueous solution of Hydrogen chloride (HCl) gas. It can be purchased from many suppliers.
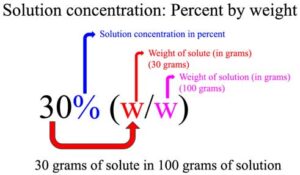
The “%” refers to solution concentration in percentage and “(w/w)” refers to solute and solution amount given in grams (i.e., percentage by weight). This means a 30% (w/w) hydrochloric acid contains 30 g of HCl per 100 g of solution.
The density of 30% (w/w) Hydrochloric acid solution is 1.15 g/ml at 20°C which means the weight of the 1 ml of the hydrochloric acid solution is 1.15 g at 20°C.
Molarity refers to the number of moles of the solute present in 1 liter of solution. To calculate the Molarity of 30% (w/w) hydrochloric acid, you need to know how many moles of HCl are present in 1 liter of solution.
In simple words, 1 mole is equal to the molecular weight of the substance. For example, 1 mole of Hydrogen chloride is equal to 36.46 g of hydrogen chloride (Molecular Weight: 36.46). To calculate the molarity, one must first calculate how much Hydrogen chloride is present in 1 L of 30% Hydrochloric acid. Once we know the amount of HCl present in 1 L solution, we can calculate the molarity of the solution by dividing the HCl amount by the molecular weight.

Calculation procedure: Molarity of 30% (w/w) Hydrochloric acid (HCl)
| Known values | |
| Molar mass of Hydrogen chloride | 36.46 g/mole |
| Concentration of Hydrochloric acid | 30 % (% by mass, w/w) |
| Density of 37% Hydrochloric acid | 1.15 g/ml |
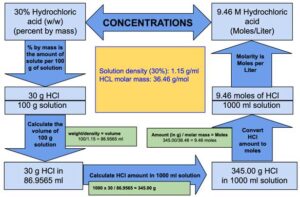
Step 1: Calculate the volume of 100 grams of Hydrochloric acid.
Formula:
Density = Weight/Volume
OR
Volume = Weight/Density
The volume of 100 g of Hydrochloric acid: 100/1.15 = 86.9565 ml
Note: 30% (w/w) hydrochloric acid means 100 g of solution contains 30 g of HCl.
The volume of 100 g of Hydrochloric acid is 86.9565 ml which means 30 g of HCl is present in 86.9565 ml of Hydrochloric acid.
Step 2: Calculate how many grams of HCl is present in 1000 ml of Hydrochloric acid.
86.9565 ml of Hydrochloric acid contains = 30 grams of HCl
1 ml of Hydrochloric acid will contain = 30/86.9565 grams of HCl
1000 ml of Hydrochloric acid will contain = (1000 x 30)/86.9565 = 345.00 grams of HCl
1000 ml of Hydrochloric acid will contain 345.00 grams of HCl.
Step 3: Calculate the number of moles of HCl in 345.00 grams of HCl.
36.46 g of HCl is equal to 1 mole.
1 g of HCl will be equal to 1/36.46 moles.
345.00 grams of Hydrogen chloride will be equal to = (345.00 x 1)/36.46 = 9.46 moles
Therefore, we can say that 1 liter of Hydrochloric acid contains 9.46 moles of HCl or in other words molarity of 30% (w/w) Hydrochloric acid is equal to 9.46 M.
Molarity of 30% (w/w) Hydrochloric acid (HCl)
Calculator – Calculate the molarity of concentrated Hydrochloric acid (HCl)
Hydrogen Chloride (HCl) Molecular weight: 36.46 g/mole
Concentration of Hydrochloric acid: % (wt/wt)
(Change the % (wt/wt) concentration)
Density of glacial Hydrochloric acid: g/ml
(Change the density)
Molarity of Hydrochloric acid: 9.46 M
1 thought on “Molarity of 30% (w/w) Hydrochloric Acid (HCl)”