Molarity of 37% (w/w) Hydrochloric Acid (HCl)
A 37% (w/w) concentrated Hydrochloric acid is a clear colorless liquid. It is an aqueous solution of hydrogen chlori (HCl). The 100 g of 37% (w/w) Hydrochloric acid contains 37 g of HCl. To calculate the molarity, one must first calculate how much HCl is present in 1 L of 37% Hydrochloric acid solution. Once we know the amount of HCl present in 1 L solution, we can calculate the molarity of the solution by dividing the HCl amount by the HCl molecular weight.
Protocol: Running DNA Samples in Agarose Gel
Agarose gel is placed in an electrophoresis tank filled with an electrophoresis buffer. DNA samples are mixed with DNA loading dye and loaded onto the wells of agarose gel. Electrophoresis apparatus is connected to electric supply and electrophoresis is performed at constant voltage until the desired separation among DNA fragments is achieved. After the run is over, gel is analyzed using UV-transilluminator or Gel Doc system.
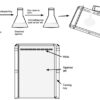
Protocol: Preparation of Agarose Gel for DNA Analysis
Agarose Gel is a gelatin-like slab, which contains small wells for loading DNA samples. It is prepared by melting agarose in a suitable electrophoresis buffer. Molten agarose is then poured into a specialized tray (casting tray), which controls the size and shape of the gel. The comb is used to create wells in agarose gel.
DNA Loading Dye
DNA sample is mixed with DNA loading dye (also called sample loading dye) prior to loading onto the wells of agarose gel for electrophoresis. DNA loading dyes contain a high-density reagent and tracking dyes. DNA loading dye makes the DNA sample heavier and coloured, thus helping the DNA sample to sink into the agarose wells without diffusing out and enabling us to monitor the loading process. In addition, tracking dyes in loading dye migrate as a diffuse band to the same direction as DNA which can be seen visually thus allowing us to monitor the progression of electrophoresis. However, tracking dyes sometimes interfere in the analysis of DNA bands by masking it.
Preparation of 6X DNA Loading Dye (Bromophenol blue, Xylene Cyanol FF, Ficoll 400)
10 ml of 6x DNA loading dye containing Bromophenol blue, Xylene cyanol FF, and Ficoll 400 is prepared by dissolving 25 mg bromophenol blue, 25 mg xylene cyanol FF and 1.5 g Ficoll 400 in water to a final volume of 10 ml. The final solution (6x DNA loading dye) will contain 0.25% (w/v) bromophenol blue, 0.25% (w/v) xylene cyanol FF, and 15% (w/v) Ficoll 400.
Preparation of 0.5 M EDTA Solution from Disodium EthyleneDiamineTetraacetate Dihydrate (EDTA.Na2.2H2O)
EDTA’s ability to sequester divalent cations from solution without any precipitation makes it a suitable choice to stop an ongoing enzymatic reaction or protect DNA from degradation. Disodium salt of EDTA (EDTA.Na2.2H2O) is most commonly used to prepare 0.5 M EDTA stock solution. A 0.5 M EDTA solution can be prepared by dissolving 186.12 grams of EDTA.Na2.2H2O (Molecular Weight: 372.24) in water to a final volume of 1000 ml.
Protocol – Passaging/Subculturing Suspension Culture
Suspension culture is passaged by diluting the existing culture. Since cells float in the medium in suspension culture, they are not treated with a trypsin-EDTA solution. To subculture a suspension cell line, a small amount of cell suspension from the existing culture is transferred to a culture dish containing fresh growth medium.