OVERVIEW
- A 10M sodium hydroxide (NaOH) stock solution is used for many applications including adjusting the pH of various solutions.
- The Normality of NaOH solution is equal to the molarity of the solution. This means that the normality of a 10M solution of NaOH is equal to 10N.
- The molecular weight of NaOH is 40. This means you need to dissolve 40 g of NaOH in water to obtain a 1 liter of 1M (or 1N) NaOH solution. To prepare a 10M NaOH solution, you need to dissolve 10 times more NaOH i.e., 400 g of NaOH for 1 L solution.
- Here we will prepare 100 ml of 10M NaOH solution. Therefore, we need to take 40 g of NaOH.
- Dissolution of NaOH is a hyperthermic reaction (generates heat), therefore, NaOH is added in water in small quantities instead of adding all at a time to limit the heat generation as excessive heat can damage the container (glass beaker).
Requirements
Reagents and solutions
Sodium hydroxide (NaOH) pallet
Deionized / Double distilled water
Equipment and disposables
Measuring cylinder/volumetric flask
Erlenmeyer flask / Beaker
Glass rod/Magnetic stirrer
Ice bucket with ice (optional)
Weighing Balance
Composition
10 M NaOH solution
Objective
Preparation of 100 ml of 10 M NaOH solution in water
Preparation
Use personal protective equipment (lab coat, gloves, goggles etc) for your safety and follow the guidelines of your institute.
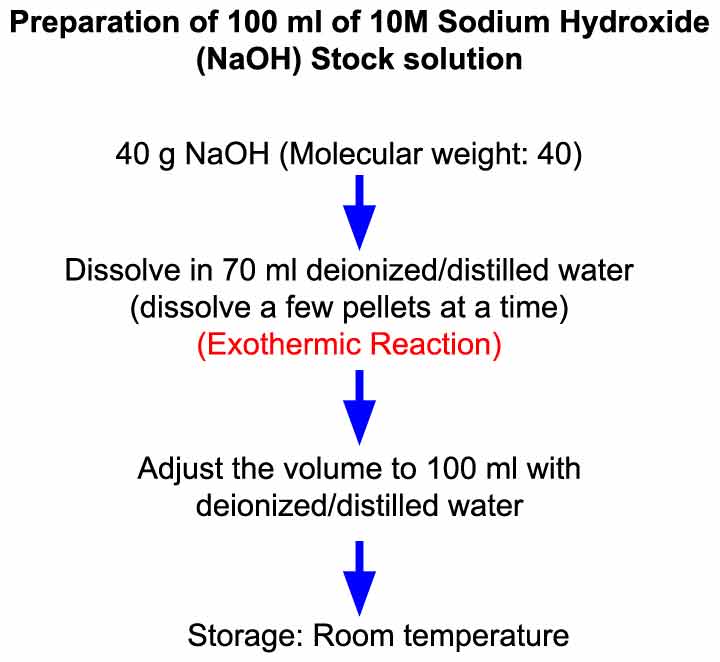
Step 1: To prepare 100 ml of 10 M NaOH solution, weigh out 40 g of NaOH (molecular weight: 40).
Precaution:
Don’t expose solid NaOH to air for a long time while weighing. NaOH is hygroscopic in nature and absorbs water from the atmosphere quickly.
Step 2: Dissolve NaOH pellets in 70 ml water by adding small quantities of NaOH in water.
◊ Take 70 ml of deionized/double distilled water in a beaker.
◊ Add a few pellets (4 – 5 pellets each time) of NaOH in water and wait until they dissolve. Repeat this until all 40 g NaOH pellets are dissolved in water.
Precautions:
1. The dissolution of NaOH is a highly exothermic reaction, which can even break the glass beakers. It is recommended to keep the beaker on ice while dissolving NaOH.
2. Never add 70 ml water to 40 g NaOH pellet.
Step 3: Adjust the solution volume to 100 ml with deionized/double distilled water.
◊ Transfer solution to a measuring cylinder/volumetric flask (capacity: 100 ml) and adjust the volume to 100 ml with deionized/double distilled water.
Storage:
The solution can be stored at room temperature for several months.
Note:
There is no need to sterilize this solution by autoclaving. No microorganism will grow in concentrated NaOH solution.
Applications
● NaOH is a strong base and is commonly used to adjust the pH of many solutions.
● Plasmid isolation by alkaline lysis method
● Preparation of EDTA solution
| Follow the table to prepare NaOH solution of specific concentration and volume | ||||
| Conc. / Volume | 50 ml | 100 ml | 250 ml | 500 ml |
| 1 M | 2.00 g | 4.00 g | 10.00 g | 20.00 g |
| 2 M | 4.00 g | 8.00 g | 20.00 g | 40.00 g |
| 5 M | 10.00 g | 20.00 g | 50.00 g | 100.00 g |
| 10 M | 20.00 g | 40.00 g | 100.00 g | 200.00 g |
CALCULATOR
Use calculator to calculate the quantities of NaOH for the preparation of NaOH solution of specific concentration and volume
Molecular weight of NaOH: 40
Volume of Sodium hydroxide solution: ml
(change the volume of the solution)
Molarity of Sodium hydroxide solution: M
(Change the molarity of the solution)
Amount of sodium hydroxide: 40 g
4 thoughts on “Preparation of 10 M Sodium Hydroxide (NaOH) Solution”