OVERVIEW

- A 50% (w/w) concentrated Sodium hydroxide solution is a clear colorless liquid. It can be purchased from many suppliers.
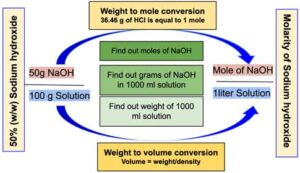
- A 50% (w/w) sodium hydroxide solution means that there is 50 g of NaOH per 100 g of solution.
- The density of 50% (w/w) Sodium hydroxide solution is 1.515 g/ml at 25°C which means that the weight of the 1 ml of Sodium hydroxide solution is 1.515 g at 25°C.
- Molarity refers to the number of moles of the solute present in 1 liter of solution.
- In simple words, 1 mole is equal to the atomic weight of the substance. For example, 1 mole of Sodium hydroxide is equal to 40.00 grams of Sodium hydroxide (NaOH, molecular weight = 40.00).
- To calculate the molarity, one must first calculate how much Sodium hydroxide is present in 1 L of 50% Sodium hydroxide solution. Once we know the amount of sodium hydroxide present in 1 L solution, we can calculate the molarity of the solution by dividing the NaOH amount by the molecular weight.
- Know more about Sodium Hydroxide
- Preparation of 10 M Sodium Hydroxide (NaOH) Solution
- Sodium Hydroxide (NaOH) Molecular Weight Calculation
- Suppliers: 50% Sodium Hydroxide Solution
Calculation procedure:
| Known values | |
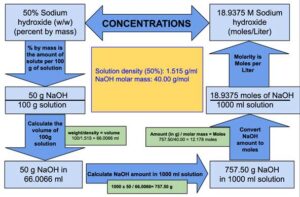
| Molar mass of Sodium Hydroxide | 40.00 g/mole |
| Concentration of Sodium Hydroxide solution | 50% (% by mass, w/w) |
| Density of 50% (w/w) Sodium Hydroxide solution | 1.515 g/ml |
Step 1: Calculate the volume of 100 grams of Sodium Hydroxide solution.
Formula:
Density =
OR
Volume =
The volume of 100 g of Sodium hydroxide solution: = 66.0066 ml
Note: 50% (w/w) Sodium Hydroxide means that 100 g of solution contains 50 g of Sodium Hydroxide.
The volume of 100 g of Sodium hydroxide solution is 66.0066 ml which means 50 g of NaOH is present in 66.0066 ml of Sodium hydroxide solution.
Step 2: Calculate how many grams of NaOH is present in 1000 ml of Sodium hydroxide solution.
66.0066 ml of Sodium Hydroxide solution contains = 50 grams of NaOH
1 ml of Sodium hydroxide solution will contain = grams of NaOH
1000 ml of Sodium hydroxide solution will contain = = 757.50 grams of NaOH
1000 ml of Sodium hydroxide solution will contain 757.50 grams of NaOH.
Step 3: Calculate the number of moles of NaOH present in 757.50 grams of NaOH.
40 g of NaOH is equal to 1 mole.
1 g of NaOH will be equal to moles.
Therefore, we can say that 1 liter of sodium hydroxide solution contains 18.9375 moles or in other words molarity of 50% (w/w) Sodium Hydroxide is equal to 18.9375 M.
Calculator – Calculate the molarity of concentrated Sodium Hydroxide (NaOH)
Use Calculator to calculate the molarity of concentrated Sodium Hydroxide (NaOH) when concentration is given in % by mass (w/w)
Sodium Hydroxide (NaOH) Molecular weight: 40.00 g/mole
Concentration of Sodium Hydroxide in % (wt/wt) : % (wt/wt)
(Change the % (wt/wt) concentration)
Density of glacial Sodium Hydroxide: g/ml
(Change the density)
Molarity of Sodium Hydroxide: 18.938 M
Ok so you lost me on a couple of specifics. I get all the calculations. However, it seems to me when it’s stated “ Calculate the volume of 100 grams of Sodium Hydroxide solution”, the solution concentration has to be assumed at the 50% w/w. And by converting it to a volume, to me at least, you are now calculating for a 50% v/v solution. I, mistakenly, would have said a 50% w/w solution would be 50g NaOH in 50ml (50g) of water. So 498.6g NaOH + 498.6g H₂O (H₂O = 0.9973g/ml @75℉, 1atm). The molarity is 498.6/40=12.46 moles in…(ok, now I get it). I was going to say, in 997.3 ml of solution which would be 12.5 molar. But you need the density to determine total volume, or grams/liter. Never mind. And thanks
40 g NaOH solid in 100 mL solution is 10% w/v. 40g/(40 g/mol) = 1 mol / 0.1 L = 10%.