OVERVIEW
A 37% (w/w) hydrochloric acid can be purchased from several suppliers. It is a clear colorless liquid and can be diluted to prepare solutions of known concentrations. Remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1M solution is also a 1N solution. Here we describe a procedure to prepare 1M hydrochloric acid solution by diluting a 37% (w/w) concentrated hydrochloric acid solution.
REQUIREMENTS
Reagents and solutions
37% (w/w) hydrochloric acid stock solution
Distilled/deionized water
Equipment and disposables
Measuring cylinder/volumetric flask
Beaker (1 Liter)
Magnetic stirrer
OBJECTIVE
Preparation of 1 Liter of 1M Hydrochloric Acid using Concentrated Stock Solution (37%, w/w)
PROCEDURE: Preparation of 1M Hydrochloric Acid From Concentrated Stock Solution (37%, w/w)
Use personal protective equipment (lab coat, gloves, goggles, etc) for your safety and follow the guidelines of your institute.
PART I: Calculate how much 37% (w/w) concentrated hydrochloric acid stock solution is required to prepare 1 L of 1 M hydrochloric acid solution
Step 1: Calculate the molarity of 37% (w/w) concentrated hydrochloric acid solution
The procedure to calculate the molarity of 37% (w/w) concentrated hydrochloric acid solution is described in detail in the post “Molarity of 37% (w/w) Hydrochloric Acid (HCl)”. From this post, you will know that the molarity of 37% (w/w) concentrated hydrochloric acid solution is 12.178M.
Step 2: Calculate how much stock solution is required to prepare 1L of 1M hydrochloric acid solution using the dilution method.
Dilution method is described in detail in the post “Preparing Working Solution from Stock Solution by Dilution”.
Use the following formula to calculate the volume of concentrated solution required for the preparation of 1 liter of 1M solution of hydrochloric acid solution
Formula: Cf x Vf = CS x VS
Cf (concentration of diluted (final) solution) = 1M
Vf (volume of diluted (final) solution) = 1 liter
CS (concentration of stock solution) = 12.178 M
VS (volume of stock solution) = ? (unknown)
Place all values in the formula:
1 x 1 = 12.178 x VS
VS = 1x 1/ 12.178 = 0.082 liter (82 ml)
So you need 0.082 liter of 37% (w/w) concentrated hydrochloric acid solution to prepare a 1 liter of 1M hydrochloric acid solution.
Step 3: Calculate the volume of solvent to be taken.
Final volume of diluted solution = 1 Liter
Volume of concentrated solution = 0.082 liter (82 ml)
Volume of solvent (water) required = 1 – 0.082 = 0.918 liter (918 ml)
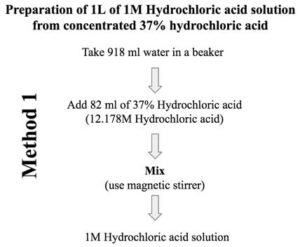
So you need to mix 0.082 liter (i.e., 82 ml) of concentrated solution to 0.918 liter (918 ml) water to prepare 1 liter of 1M hydrochloric acid solution.
PART II: Solution preparation
Step 4: Transfer 918 ml water in a beaker/volumetric flask/measuring cylinder. Add 82 ml of 37% concentrated hydrochloric acid stock solution and mix it to prepare a homogeneous solution.
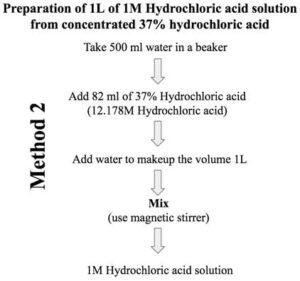
Note: In case you have not calculated the amount of water to be added, just take ≈ 500 ml water in a volumetric flask/measuring cylinder (capacity 1L) and add 82 ml of 37% concentrated hydrochloric acid stock solution. Now adjust the final volume to 1 liter with water. Mix it.
STORAGE
Solution can be stored at room temperature.
1 thought on “Preparation of 1M Hydrochloric Acid From Concentrated Stock Solution (37%, w/w)”