Overview
- Cryopreservation (Cryo: icy cold or frost; Preservation: Storage) is an efficient way of preserving cell culture at ultra-low temperatures below -135°C. Preserved cells can be revived whenever needed.
- Preserving cells at low temperatures below the freezing point is very stressful to the cells and can cause cell damage and death. The cryopreservation procedure ensures cells survive the stress and damaging effects of freezing and remain viable.
- Cryopreservation not only stops biological time and aging but also protects cell culture from accidental loss due to mishandling and contamination.
- Serum-containing cryopreservative medium is used to preserve cell lines that are maintained in the serum-containing growth medium while serum-free cryopreservative medium is used for cell lines that are maintained in serum-free chemically defined culture medium.
- Serum-containing cryopreservative medium (also known as freezing medium) can contain high concentrations of serum (20 – 90%) and DMSO (5 – 15%) in the growth medium.
- The composition of the cryopreservative medium (serum concentration, cryoprotective agent, and its concentration), rate of cooling during the freezing process, and rate of warming up during the revival/thawing process are the critical parameters that determine the success of the cryopreservation process.
- A high concentration of serum, slow cooling (during freezing), and rapid warming (during revival) are generally accepted as good cryopreservative conditions as these conditions protect cells from damage induced by stressful cryopreservation processes.
- A slow cooling rate reduces the formation of intracellular ice crystals by allowing the water to escape from cells (dehydration).
- DMSO, a cryoprotectant, protects cells from death by preventing ice crystal formation. High serum content also protects cells from death during cryopreservation and revival.
Overview of the procedure
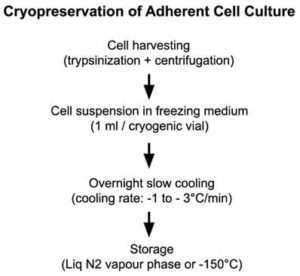
Requirements
Reagents
♦ Growth medium (e.g., DMEM supplemented with 10% FBS)
♦ PBS (Ca2+-free and Mg2+-free)
♦ Trypsin EDTA solution (or a suitable cell dissociation reagent)
♦ Freezing medium (50% growth medium + 20% FBS + 10% DMSO)
Equipment and disposables
♦ Sterile cryogenic vials
♦ Sterile conical tubes (15 mL or 50 mL)
♦ Controlled rate freezing apparatus
♦ Haemocytometer/Trypan Blue
♦ -80°C freezer
♦ Liquid nitrogen storage container/-150°C freezer
♦ Benchtop centrifuge with 45° fixed-angle or swinging-bucket rotor (e.g., Eppendorf™ 5804 Series)
♦ Personal protective equipment (sterile gloves, laboratory coat, Full-face protective mask/visor)
♦ Laminar flow hood
♦ Pipette tips and pipetman
♦ Serological pipettes and Pipetboy
♦ Inverted phase-contrast microscope
Starting materials
Late log-phase healthy monolayer culture (80% – 90% confluent culture)
Tip: Change the medium the previous day to remove any cell debris present. This will also help you to keep the culture healthy and to examine the culture for contamination.
Protocol
Prior to start
1. Visually inspect the cell culture carefully under an inverted phase-contrast microscope and make sure that cells are healthy and do not have any contamination.
2. Properly label Cryogenic vials. You must write clearly the cell line name, passage number, and date on cryogenic vials.
Note: Here we describe a general procedure for cryopreserving adherent cell culture. For specific details, we recommend you to carefully read the manual provided with the cell line or consult the supplier.
Step 1: Harvest cells from the culture using a standard procedure (trypsinization)
> Discard the culture medium and wash the monolayer with PBS. Add sufficient amounts of trypsin EDTA solution (1 -2 ml for a T25 flask) and incubate at 37°C for 1 – 2 min. Inspect the dish under the microscope for cell detachment. Incubate at 37°C for some more time if cells are not dislodged.
> Once the cells are dislodged, tap the flask/dish 2-3 times to make sure all the cells have come out from the dish surface. Add serum-containing growth medium (4 ml for a T25 flask) and flush the entire surface of the dish by pipetting to collect all cells from the dish.
> Transfer cell suspension to a sterile centrifuge tube. Cells from two or more dishes from the same passage number (subculture) can be combined in one tube.
Note:
Serum in the complete growth medium has trypsin inactivating activity.
Tip:
At this point, cells can be collected by centrifugation and the cell pellet can be resuspended in a sufficient amount of growth medium. This step is not necessary. If you suspect high cell death during the trypsinization process, or if the cells are too diluted in suspension, centrifugation and resuspension of cells can be done.
Caution:
Make sure that the trypsinization of cells does not cause enormous cell death. Healthy cells are a prerequisite for cryopreservation.
Step 2: Determine viable cell density in cell suspension (optional)
> Determine viable cell numbers using trypan blue exclusion assay and a hemocytometer.
> To count viable cells, mix 20 µl cell suspension with 20 µl trypan blue solution. Place the solution onto the hemocytometer chamber.
> Count live and dead cells. Calculate viable cell count per ml and percentage of dead cells.
> Viability over 90% is considered a healthy culture.
Tips
1. You can use other sophisticated methods like Countess® Automated Cell Counter or Moxi Flow Kit which can determine viability cell count quickly.
2. If cells are very diluted, collect cells by centrifugation and resuspend the cell pellet in an appropriate amount of growth medium.
3. In many cases, based on previous experience you can determine how many cryovials can be frozen from a culture dish. One can prepare 2- 4 ml cell suspension in a freezing medium from a near-confluent T25 flask which can be used to prepare 2 – 4 cryogenic vials (1 ml/vial).
4. Counting is required when cell number is limited and you want to freeze as many cryogenic vials as possible e.g., primary culture.
Cautions
1. Make sure that cells are properly resuspended before counting viable cells.
2. Don’t use cells if cell death is high. In this case, cells can be plated in fresh culture dishes that can be used for cryopreservation when they are ready.
Step 3: Resuspend the cells in an ice-cold freezing medium at the recommended viable cell density (1 x 106 – 5 x 106 cells/ml)
> Harvest cells from cell suspension by centrifugation at 4°C for 5 – 10 min at 250 × g (1000 – 1500 rpm for Eppendorf™ 5804 Series benchtop centrifuge).
> Carefully aspirate supernatant as much as possible without disturbing the cell pellet.
> Flick the tube with your finger several times to dislodge the pellet.
> Add an appropriate volume of ice-cold freezing medium to obtain the right viable cell density (between 1 x 106 – 5 x 106 cells/ml). Resuspend the cells thoroughly with gentle pipetting.
Tip
Cell density in the freezing medium can vary with cell lines. In most cases, high cell density is good for cell recovery.
Cautions
1. Since DMSO can be toxic to cells, it is advisable to use a chilled freezing medium. Try your best to maintain the temperature of cell suspension at 4°C.
2. Quickly resuspend the pellet in the freezing medium immediately after aspirating supernatant.
3. Centrifugation speed should be sufficient to get a soft pellet. The pellet should not be too tight. A tight pellet will be difficult to resuspend and attempts to resuspend it by vigorous pipetting may cause cell death.
Step 4: Aliquot cell suspension into cryogenic vials
> Place cryovials on ice.
> Transfer 1 ml aliquots of cell suspension into cryovials.
> Tighten caps on vials immediately.
Caution
While aliquoting, frequently, and gently mix the cells to maintain a homogeneous cell suspension.
Step 5: Subject cryovials to slow cooling (1 – 3°C/min) overnight in -80°C freezer
> Place all cryogenic vials in a controlled rate freezing apparatus (e.g., CoolCell® Cell Freezing Containers from Biocision) and immediately store in -80°C freezer overnight.
Tips
1. The most efficient way to freeze cryogenic vials is to use controlled-rate freezers (e.g., CryoMed™ Controlled-Rate Freezers from ThermoFisher Scientific). However, for most serum-grown cell lines, one can use commercial cooling devices e.g., CoolCell® Cell Freezing Containers from Biocision, or Mr. Frosty™ Freezing Container from ThermoFisher Scientific.
2. Homemade cooling devices – A Thermocol box (small size) filled with cotton or tissue paper, can also be used in place of commercial cooling devices.
Step 6: Store cryogenic vials in liquid nitrogen
> Transfer all frozen cryogenic vials to a liquid nitrogen container the next day. Alternatively, frozen cryogenic vials can also be stored in a -150°C freezer.
> Cryogenic vials can be stored in the gas phase or liquid phase of liquid nitrogen.
Tip
A frozen vial can be revived after 2 weeks to ensure that frozen stocks are viable and free of any contamination.
Cautions
1. Biosafety level 2 cell lines should be stored in the gas phase of liquid nitrogen.
2. You must maintain the proper records of the location of frozen cell lines.
3. Wear protective equipment when handling liquid nitrogen. Remember that cryogenic vials may explode if they are stored in a liquid phase of liquid nitrogen.
2 thoughts on “Protocol – Cryopreservation of Adherent Cell Culture”