The molecular weight of Sodium phosphate dibasic dodecahydrate [Na2HPO4.12H2O] is 358.1437.
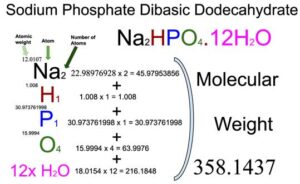 Sodium phosphate dibasic (Na2HPO4), also called Disodium phosphate (DSP) or disodium hydrogen phosphate, is an inorganic compound of four elements: Sodium, Hydrogen, Phosphorus, and Oxygen. The dodecahydrate form of Sodium phosphate dibasic (Na2HPO4.12H2O) also contains 12 water molecules. The molecular weight of Sodium phosphate dibasic dodecahydrate is 358.1437 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements and water molecules.
Sodium phosphate dibasic (Na2HPO4), also called Disodium phosphate (DSP) or disodium hydrogen phosphate, is an inorganic compound of four elements: Sodium, Hydrogen, Phosphorus, and Oxygen. The dodecahydrate form of Sodium phosphate dibasic (Na2HPO4.12H2O) also contains 12 water molecules. The molecular weight of Sodium phosphate dibasic dodecahydrate is 358.1437 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements and water molecules.
CALCULATION PROCEDURE: Sodium phosphate dibasic dodecahydrate [Na2HPO4.12H2O] Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in a Sodium phosphate dibasic dodecahydrate molecule.
From the chemical formula, you will know different atoms and their number in a Sodium phosphate dibasic dodecahydrate molecule. The chemical formula of Sodium phosphate dibasic dodecahydrate is Na2HPO4.12H2O. From the chemical formula, you can find that one molecule of Sodium phosphate dibasic dodecahydrate consists of two Sodium (C) atoms, one Hydrogen (H) atom, one Phosphorus (P) atom, four Oxygen atoms, and twelve water molecules.
Step 2: Find out the atomic weights of each atom (from the periodic table).
Atomic weight of Sodium (Na): 22.98976928 (Ref: Ciaaw-sodium)
Atomic weight of Hydrogen (H): 1.008 (Ref: Lanl-1)
Atomic weight of Oxygen (O): 15.9994 (Ref: Jlab-ele008)
Atomic weight of Phosphorus (P): 30.973761998 (Ref: Jlab-ele015)
Molecular weight of water: 18.0154
Step 3: Calculate the total weight of each atom in a Sodium phosphate dibasic dodecahydrate molecule by multiplying its atomic weight by its number.
Number of Sodium atoms in Sodium phosphate dibasic dodecahydrate: 2
Atomic weight of Sodium (Na): 22.98976928
Total weight of Sodium atoms in Sodium phosphate dibasic dodecahydrate: 22.98976928 x 2 = 45.97953856
Number of Hydrogen atoms in Sodium phosphate dibasic dodecahydrate: 1
Atomic weight of Hydrogen (H): 1.008
Total weight of Hydrogen atoms in Sodium phosphate dibasic dodecahydrate: 1.008 x 1 = 1.008
Number of Phosphorus atoms in Sodium phosphate dibasic dodecahydrate: 1
Atomic weight of Phosphorus (P): 30.973761998
Total weight of Phosphorus atoms in Sodium phosphate dibasic dodecahydrate: 30.973761998 x 1 = 30.973761998
Number of Oxygen atoms in Sodium phosphate dibasic dodecahydrate: 4
Atomic weight of Oxygen (O): 15.9994
Total weight of Oxygen atoms in Sodium phosphate dibasic dodecahydrate: 15.9994 x 4 = 63.9976
Number of water (H2O) molecules in Sodium phosphate dibasic dodecahydrate: 12
Molecular weight of water: 18.0154
Total weight of water in Sodium phosphate dibasic dodecahydrate: 18.0154 x 12 = 216.1848
Step 4: Calculate the molecular weight of Sodium phosphate dibasic dodecahydrate by adding up the total weight of all atoms.
Molecular weight of Sodium phosphate dibasic dodecahydrate: 45.97953856 (Sodium) + 1.008 (Hydrogen) + 30.973761998 (Phosphorus) + 63.9976 (Oxygen) + 216.1848 (Water) = 358.1437
So the molecular weight of Sodium phosphate dibasic dodecahydrate is 358.1437.
Sodium phosphate dibasic dodecahydrate [Na2HPO4.12H2O] Molecular Weight Calculation
| Molecular weight of Sodium phosphate dibasic dodecahydrate [Na2HPO4.12H2O] | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| Sodium (Na) | 2 | 22.98976928 | 45.97953856 |
| Hydrogen (H) | 1 | 1.008 | 1.008 |
| Phosphorus (P) | 1 | 30.973761998 | 30.973761998 |
| Oxygen (O) | 4 | 15.9994 | 63.9976 |
| Water (H2O) | 12 | 18.0154 | 216.1848 |
| Molecular weight of Sodium phosphate dibasic dodecahydrate [Na2HPO4.12H2O]: | 358.1437 | ||
REFERENCES:
- Lanl-1: https://periodic.lanl.gov/1.shtml
- Ciaaw-sodium: https://www.ciaaw.org/sodium.htm
- Jlab-ele008: https://education.jlab.org/itselemental/ele008.html
- Jlab-ele015: https://education.jlab.org/itselemental/ele015.html