The molecular weight of Sodium phosphate dibasic [Na2HPO4] is 141.9589.
 Sodium phosphate dibasic (Na2HPO4), also called Disodium phosphate or disodium hydrogen phosphate, is an inorganic compound of four elements: Sodium, Hydrogen, Phosphorus, and Oxygen. The molecular weight of Sodium phosphate dibasic is 141.9589 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements.
Sodium phosphate dibasic (Na2HPO4), also called Disodium phosphate or disodium hydrogen phosphate, is an inorganic compound of four elements: Sodium, Hydrogen, Phosphorus, and Oxygen. The molecular weight of Sodium phosphate dibasic is 141.9589 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements.
CALCULATION PROCEDURE: Sodium phosphate dibasic [Na2HPO4] Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in a Sodium phosphate dibasic molecule.
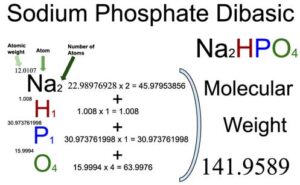
From the chemical formula, you will know different atoms and their number in a Sodium phosphate dibasic molecule. The chemical formula of Sodium phosphate dibasic is Na2HPO4. From the chemical formula of Sodium phosphate dibasic, you can find that one molecule of Sodium phosphate dibasic consists of two Sodium (C) atoms, one Hydrogen (H) atom, one Phosphorus (P) atom, and four Oxygen atoms.
Step 2: Find out the atomic weights of each atom (from the periodic table).
Atomic weight of Sodium (Na): 22.98976928 (Ref: Ciaaw-sodium)
Atomic weight of Hydrogen (H): 1.008 (Ref: Lanl-1)
Atomic weight of Oxygen (O): 15.9994 (Ref: Jlab-ele008)
Atomic weight of Phosphorus (P): 30.973761998 (Ref: Jlab-ele015)
Step 3: Calculate the total weight of each atom in a Sodium phosphate dibasic molecule by multiplying its atomic weight by its number.
Number of Sodium atoms in Sodium phosphate dibasic: 2
Atomic weight of Sodium (Na): 22.98976928
Total weight of Sodium atoms in Sodium phosphate dibasic: 22.98976928 x 2 = 45.97953856
Number of Hydrogen atoms in Sodium phosphate dibasic: 1
Atomic weight of Hydrogen (H): 1.008
Total weight of Hydrogen atoms in Sodium phosphate dibasic: 1.008 x 1 = 1.008
Number of Phosphorus atoms in Sodium phosphate dibasic: 1
Atomic weight of Phosphorus (P): 30.973761998
Total weight of Phosphorus atoms in Sodium phosphate dibasic: 30.973761998 x 1 = 30.973761998
Number of Oxygen atoms in Sodium phosphate dibasic: 4
Atomic weight of Oxygen (O): 15.9994
Total weight of Oxygen atoms in Sodium phosphate dibasic: 15.9994 x 4 = 63.9976
Step 4: Calculate the molecular weight of Sodium phosphate dibasic by adding up the total weight of all atoms.
Molecular weight of Sodium phosphate dibasic: 45.97953856 (Sodium) + 1.008 (Hydrogen) + 30.973761998 (Phosphorus) + 63.9976 (Oxygen) = 141.9589
So the molecular weight of Sodium phosphate dibasic is 141.9589.
Sodium phosphate dibasic [Na2HPO4] Molecular Weight Calculation
| Molecular weight of Sodium phosphate dibasic [Na2HPO4] | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| Sodium (Na) | 2 | 22.98976928 | 45.97953856 |
| Hydrogen (H) | 1 | 1.008 | 1.008 |
| Phosphorus (P) | 1 | 30.973761998 | 30.973761998 |
| Oxygen (O) | 4 | 15.9994 | 63.9976 |
| Molecular weight of Sodium phosphate dibasic [Na2HPO4]: | 141.9589 | ||
REFERENCES:
- Lanl-1: https://periodic.lanl.gov/1.shtml
- Ciaaw-sodium: https://www.ciaaw.org/sodium.htm
- Jlab-ele008: https://education.jlab.org/itselemental/ele008.html
- Jlab-ele015: https://education.jlab.org/itselemental/ele016.html