The molecular weight of Acesulfame potassium (C4H4KNO4S) is 201.24344.
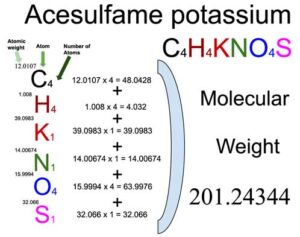 Acesulfame potassium (C4H4KNO4S) is an organic compound of six elements: Carbon, Hydrogen, Potassium, Nitrogen, Oxygen and Sulfur. The molecular weight of Acesulfame potassium (C4H4KNO4S) is 201.24344 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Carbon, Hydrogen, Potassium, Nitrogen, Oxygen and Sulfur.
Acesulfame potassium (C4H4KNO4S) is an organic compound of six elements: Carbon, Hydrogen, Potassium, Nitrogen, Oxygen and Sulfur. The molecular weight of Acesulfame potassium (C4H4KNO4S) is 201.24344 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Carbon, Hydrogen, Potassium, Nitrogen, Oxygen and Sulfur.
CALCULATION PROCEDURE: Acesulfame potassium [C4H4KNO4S] Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in an Acesulfame potassium molecule.
You will know different atoms and their number in an Acesulfame potassium molecule from the chemical formula. The chemical formula of Acesulfame potassium is C4H4KNO4S. From the chemical formula, you can find that one molecule of Acesulfame potassium consists of four Carbon (C) atoms, four Hydrogen (H) atoms, one Potassium (K) atom, one Nitrogen (N) atom, four Oxygen (O) atoms, and one Sulfur (S) atom.
Step 2: Find out atomic weights of each atom (from periodic table).
Atomic weight of Carbon (C): 12.0107 (Ref: Jlab-ele006)
Atomic weight of Hydrogen (H): 1.008 (Ref: Lanl-1)
Atomic weight of Potassium (K): 39.0983 (Ref: Ciaaw-potassium)
Atomic weight of Nitrogen (N): 14.00674 (Ref: Jlab-ele007)
Atomic weight of Oxygen (O): 15.9994 (Ref: Jlab-ele008)
Atomic weight of Sulfur (S): 32.066 (Ref: Jlab-ele016)
Step 3: Calculate the molecular weight of Acesulfame potassium by adding the total weight of all atoms.
Number of Carbon (C) atoms in Acesulfame potassium: 4
Atomic weight of Potassium: 12.0107
Total weight of Carbon atoms in Acesulfame potassium: 12.0107 x 4 = 48.0428
Number of Hydrogen (H) atoms in Acesulfame potassium: 4
Atomic weight of Hydrogen: 1.008
Total weight of Hydrogen atoms in Acesulfame potassium: 1.008 x 4 = 4.032
Number of Potassium (K) atoms in Acesulfame potassium: 1
Atomic weight of Potassium: 39.0983
Total weight of Potassium atoms in Acesulfame potassium: 39.0983 x 1 = 39.0983
Number of Nitrogen (N) atoms in Acesulfame potassium: 1
Atomic weight of Nitrogen: 14.00674
Total weight of Nitrogen atoms in Acesulfame potassium: 14.00674 x 1 = 14.00674
Number of Oxygen (O) atoms in Acesulfame potassium: 4
Atomic weight of Oxygen: 15.9994
Total weight of Oxygen atoms in Acesulfame potassium: 15.9994 x 4 = 63.9976
Number of Sulfur (S) atoms in Acesulfame potassium: 1
Atomic weight of Sulfur: 32.066
Total weight of Sulfur atoms in Acesulfame potassium: 32.066 x 1 = 32.066
Step 4: Calculate the molecular weight of Acesulfame potassium by adding up the total weight of all atoms.
Molecular weight of Acesulfame potassium : 48.0428 (Carbon) + 4.032 (Hydrogen) + 39.0983 (Potassium) + 14.00674 (Nitrogen) + 63.9976 (Oxygen) + 32.066 (Sulfur)
So the molecular weight of Acesulfame potassium is 201.24344.
Acesulfame potassium [C4H4KNO4S] Molecular Weight Calculation
| Molecular weight of Acesulfame potassium (C4H4KNO4S) | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| Carbon (C) | 4 | 12.0107 | 48.0428 |
| Hydrogen (H) | 4 | 1.008 | 4.032 |
| Potassium (K) | 1 | 39.0983 | 39.0983 |
| Nitrogen (N) | 1 | 14.00674 | 14.00674 |
| Oxygen (O) | 4 | 15.9994 | 63.9976 |
| Sulfur (S) | 1 | 32.066 | 32.066 |
| Molecular weight of Acesulfame potassium (K2SO4): | 201.24344 | ||
REFERENCES:
- Lanl-1: https://periodic.lanl.gov/1.shtml
- Jlab-ele006: https://education.jlab.org/itselemental/ele006.html
- Ciaaw-potassium: https://www.ciaaw.org/potassium.htm
- Jlab-ele007: https://education.jlab.org/itselemental/ele007.html
- Jlab-ele008: https://education.jlab.org/itselemental/ele008.html
- Jlab-ele016: https://education.jlab.org/itselemental/ele016.html