The molecular weight of Calcium chloride dihydrate [CaCl2.2H2O] (CAS Registry Number: 10035-04-8) is 147.0142.
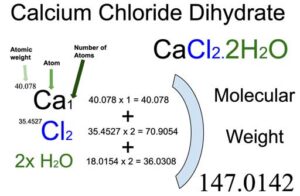 Calcium Chloride [CaCl2] is an inorganic compound of two elements: Calcium (Ca) and Chlorine (Cl). The dihydrate form of Calcium Chloride (CaCl2.2H2O) also contains 2 water (H2O) molecules. The molecular weight of Calcium chloride dihydrate is 147.0142 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements and water molecules.
Calcium Chloride [CaCl2] is an inorganic compound of two elements: Calcium (Ca) and Chlorine (Cl). The dihydrate form of Calcium Chloride (CaCl2.2H2O) also contains 2 water (H2O) molecules. The molecular weight of Calcium chloride dihydrate is 147.0142 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements and water molecules.
CALCULATION PROCEDURE: Calcium Chloride Dihydrate (CaCl2.2H2O) Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in a Calcium chloride dihydrate molecule.
You will know different atoms and their number in a Calcium chloride dihydrate molecule from the chemical formula. The chemical formula of Calcium chloride dihydrate is CaCl2.2H2O. From the chemical formula, you can find that one molecule of Calcium chloride dihydrate consists of one Calcium (Ca) atom, two Chlorine (Cl) atoms, and two molecules of water.
Step 2: Find out the atomic weights of each atom (from the periodic table).
Atomic weight of Calcium (Ca): 40.078 (Ref: Ciaaw-calcium, Jlab-ele020)
Atomic weight of Chlorine (Cl): 35.4527 (Ref: Jlab-ele017)
Molecular weight of Water [H2O]: 18.0154
Step 3: Calculate the molecular weight of Calcium chloride dihydrate by adding the total weight of all atoms.
Number of Calcium atoms in Calcium chloride dihydrate: 1
Atomic weight of Calcium: 40.078
Total weight of Calcium atoms in Calcium chloride dihydrate: 40.078 x 1 = 40.078
Number of Chlorine atoms in Calcium chloride dihydrate: 2
Atomic weight of Chlorine: 35.4527
Total weight of Chlorine atoms in Calcium chloride dihydrate: 35.4527 x 2 = 70.9054
Number of water (H2O) molecules in Calcium chloride dihydrate: 2
Molecular weight of water: 18.0154
Total weight of water in Calcium chloride dihydrate: 18.0154 x 2 = 36.0308
Step 4: Calculate the molecular weight of Calcium chloride dihydrate by adding up the total weight of all atoms.
Molecular weight of Calcium chloride dihydrate (CaCl2.2H2O): 40.078 (Calcium) + 70.9054 (Chlorine) + 36.0308 (water) = 147.0142
So the molecular weight of Calcium chloride dihydrate is 147.0142.
Calcium Chloride Dihydrate (CaCl2.2H2O) Molecular Weight Calculation
| Molecular weight of Calcium chloride dihydrate (CaCl2.2H2O) | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| Calcium (Ca) | 1 | 40.078 | 40.078 |
| Chlorine (Cl) | 2 | 35.4527 | 70.9054 |
| Water (H2O) | 2 | 18.0154 | 36.0308 |
| Molecular weight of Calcium chloride dihydrate (CaCl2.2H2O): | 147.0142 | ||
REFERENCES:
- Pubchem-20: https://pubchem.ncbi.nlm.nih.gov/element/20
- Ciaaw-calcium: https://www.ciaaw.org/calcium.htm
- Jlab-ele020: https://education.jlab.org/itselemental/ele020.html
- jlab-ele017: https://education.jlab.org/itselemental/ele017.html
- cas.org: https://commonchemistry.cas.org/detail?cas_rn=10035-04-8
RELATED POSTS
- Calcium Chloride (CaCl2) Molecular Weight Calculation
- Calcium Chloride Dihydrate (CaCl2.2H2O) Molecular Weight Calculation
- Calcium Chloride Tetrahydrate (CaCl2.4H2O) Molecular Weight Calculation
- Calcium Chloride Hexahydrate (CaCl2.6H2O) Molecular Weight Calculation
FURTHER READING
Article authenticity Index: *****
* (Newly added)
***** (Revised 4 times)
********** (Revised 9 times)
# Please let us know if you find any mistakes. Your comments are welcome in the comment box.
3 thoughts on “Calcium Chloride Dihydrate (CaCl2.2H2O) Molecular Weight Calculation”