The molecular weight of Potassium hydroxide (KOH) is 56.1053.
Potassium hydroxide (KOH) is an inorganic compound of two elements: Potassium and Oxygen. The molecular weight of Potassium hydroxide (KOH) is 39.996769 which can be calculated by adding up the atomic weight of Potassium and Oxygen.
 To calculate molecular weight of any compound, the first step is to know the constituent elements (atoms) and their number in that particular compound. Then calculate the total atomic weight of each element by multiplying its atomic weight by its number. The sum of total atomic weight of all constituent elements will be the molecular weight of the compound. Note that the value of atomic weight may differ from different sources. This is because some sources use conventional atomic weight while others use standard atomic weights. Another important point to consider is the isotopes of an element. Different isotopes of an element will have different atomic weights.
To calculate molecular weight of any compound, the first step is to know the constituent elements (atoms) and their number in that particular compound. Then calculate the total atomic weight of each element by multiplying its atomic weight by its number. The sum of total atomic weight of all constituent elements will be the molecular weight of the compound. Note that the value of atomic weight may differ from different sources. This is because some sources use conventional atomic weight while others use standard atomic weights. Another important point to consider is the isotopes of an element. Different isotopes of an element will have different atomic weights.
CALCULATION PROCEDURE: Potassium Hydroxide (KOH) Molecular Weight Calculation
Step 1: Find out the chemical formula and determine constituent atoms and their number in a Potassium hydroxide molecule.
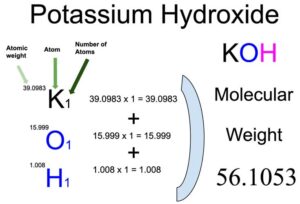
From the chemical formula, you will know different atoms and their number in a Potassium hydroxide molecule. Chemical formula of Potassium hydroxide is KOH. From the chemical formula of Potassium hydroxide, it is easy to find out that there are one Potassium (K) atom, one Oxygen (O) atom and 1 Hydrogen (H) atom in a molecule of Potassium hydroxide.
Step 2: Find out atomic weights of each atom (from periodic table).
Atomic weight of Potassium (K): 39.0983 (Ref: pubchem-Potassium)
Atomic weight of Oxygen (O) : 15.999 (Ref: Pubchem-Oxygen)
Atomic weight of Hydrogen (H) : 1.008 (Ref: Pubchem-Hydrogen)
Step 3: Calculate the total weight of each atom present in Potassium hydroxide by multiplying its atomic weight by its number.
Number of Sodium atoms in Potassium hydroxide: 1
Atomic weight of Potassium: 39.0983
Total weight of Sodium atoms in Potassium hydroxide: 39.0983
Number of Oxygen atoms in Potassium hydroxide: 1
Atomic weight of Oxygen: 15.999
Total weight of oxygen atoms in Potassium hydroxide: 15.999
Number of Hydrogen atoms in Potassium hydroxide: 1
Atomic weight of Hydrogen: 1.008
Total weight of Hydrogen atoms in Sodium hydroxide: 1.008
Step 4: Calculate the molecular weight of Potassium hydroxide by adding up the total weight of all atoms.
Molecular weight of Potassium hydroxide (KOH): 39.0983 (Potassium) + 15.999 (Oxygen) + 1.008 (Hydrogen)
So the molecular weight of Potassium hydroxide is 39.996769.
Potassium Hydroxide (KOH) Molecular Weight Calculation
| Molecular weight of Potassium hydroxide (KOH) | |||
| Constituent atoms | Number of each atom | Atomic weight | Total weight |
| K (Potassium) | 1 | 39.0983 | 39.0983 |
| O (Oxygen) | 1 | 15.999 | 15.999 |
| H (Hydrogen) | 1 | 1.008 | 1.008 |
| Molecular weight of Potassium hydroxide (KOH): | 56.1053 | ||