OVERVIEW
Freezing suspension cell culture is somewhat easier than freezing adherent cell culture as it does not require a cell detachment step (trypsinization step) from the substratum (tissue culture dish). However, in some situations such as in the presence of large tight clumps, a trypsinization step can be included to destroy the large cell clumps which can otherwise cause low yield upon revival.
To cryopreserve, cells from the suspension culture are harvested by centrifugation and resuspended at the correct cell density in an appropriate freezing medium. The resuspension is aliquoted into cryovials and subjected to slow freezing overnight followed by transfer to either the vapor phase of liquid nitrogen or a -150°C freezer.
REQUIREMENTS
Reagents
♦ Growth medium (e.g., DMEM supplemented with 10% FBS)
♦ Freezing medium (50% growth medium + 20% FBS + 10% DMSO)
Equipment and disposables
♦ Sterile cryogenic vials
♦ Sterile conical tubes (15 mL or 50 mL)
♦ Controlled rate freezing apparatus
♦ Haemocytometer/Trypan Blue
♦ -80°C freezer
♦ Liquid nitrogen storage container/-150°C freezer
♦ Benchtop centrifuge with 45° fixed-angle or swinging-bucket rotor (e.g., Eppendorf™ 5804 Series)
♦ Personal protective equipment (sterile gloves, laboratory coat, Full-face protective mask/visor)
♦ Laminar flow hood
♦ Pipette tips and pipetman
♦ Serological pipettes and Pipetboy
♦ Inverted phase-contrast microscope
Prior to start:
1. Visually inspect the cell culture carefully under an inverted phase-contrast microscope and make sure that cells are healthy and have no contamination.
2. Properly label Cryogenic vials. You must write clearly the cell line name, passage number, and date on cryogenic vials.
Note:
Here we describe a general procedure for cryopreserving suspension cell culture. For specific details, we recommend you carefully read the manual provided with the cell line or consult the supplier.
Tip:
It is recommended to change the medium of the cells the previous day. To change the medium, harvest the cells by centrifugation, resuspend the cell pellet in growth medium, and culture in appropriate culture conditions.
Precaution:
Use appropriate personal protective equipment (lab coat, gloves, goggles, etc) for your safety and follow your institute’s guidelines.
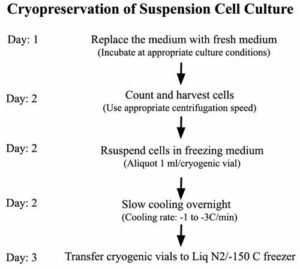
PROTOCOL
Step 1: Determine viable cell density in cell suspension (optional)
◊ Suspend the suspension culture properly by pipetting a few times up and down. This will also break the clumps.
◊ Determine viable cell numbers using trypan blue exclusion assay and a hemocytometer.
◊ To count viable cells, mix 20 µl cell suspension with 20 µl trypan blue solution. Place the solution onto the hemocytometer chamber.
◊ Count live and dead cells. Calculate viable cell count per ml and percentage of dead cells.
◊ Viability over 90% is considered a healthy culture.
Tips
1. You can use other sophisticated methods like Countess® Automated Cell Counter or Moxi Flow Kit which can determine viability cell count quickly.
2. In many cases, based on previous experience you can determine how many cryovials can be frozen from a culture dish.
Precautions
1. Make sure that cells are properly resuspended before counting viable cells.
2. Don’t use cells if cell death is high. In this case, cells can be centrifuged at an appropriate speed to remove dead cells. Harvested cells can be resuspended in a growth medium and culture for 1 day before freezing.
Step 2: Harvest cells from the culture using a standard procedure: centrifugation followed by discarding the supernatant
◊ Transfer cell suspension to a sterile centrifuge tube. Cells from two or more dishes from the same passage number (subculture) can be combined in one tube.
Step 3: Resuspend the cells in an ice-cold freezing medium at the recommended viable cell density (1 x 106 – 5 x 106 cells/ml for most mammalian tumor cell lines)
◊ Harvest cells from cell suspension by centrifugation at 4°C for 5 – 10 min at 250 × g (1000 – 1500 rpm for Eppendorf™ 5804 Series benchtop centrifuge).
◊ Carefully aspirate supernatant as much as possible without disturbing the cell pellet.
◊ Flick the tube with your finger several times to dislodge the pellet.
◊ Add an appropriate volume of ice-cold freezing medium to obtain the right viable cell density (between 1 x 106 – 5 x 106 cells/ml). Resuspend the cells thoroughly with gentle pipetting.
Tip
Cell density in the freezing medium can vary with cell lines. In most cases, high cell density is good for cell recovery.
Precautions
1. Since DMSO can be toxic to cells, it is advisable to use a chilled freezing medium. Try your best to maintain the temperature of cell suspension at 4°C.
2. Quickly resuspend the pellet in the freezing medium immediately after aspirating the supernatant.
3. Centrifugation speed should be sufficient to get a soft pellet. The pellet should not be too tight. A tight pellet will be difficult to resuspend and attempts to resuspend it by vigorous pipetting may cause cell death.
Step 4: Aliquot cell suspension into cryogenic vials
◊ Place cryovials on ice.
◊ Transfer 1 ml aliquots of cell suspension into cryovials.
◊ Tighten caps on vials immediately.
Precaution
While aliquoting, frequently, and gently mix the cells to maintain a homogeneous cell suspension.
Step 5: Subject cryovials to slow cooling (1 – 3°C/min) overnight in -80°C freezer
◊ Place all cryogenic vials in a controlled rate freezing apparatus (e.g., CoolCell® Cell Freezing Containers from Biocision) and immediately store in a -80°C freezer overnight.
Tips
1. The most efficient way to freeze cryogenic vials is to use controlled-rate freezers (e.g., CryoMed™ Controlled-Rate Freezers from ThermoFisher Scientific). However, for most serum-grown cell lines, one can use commercial cooling devices e.g., CoolCell® Cell Freezing Containers from Biocision, or Mr. Frosty™ Freezing Container from ThermoFisher Scientific.
2. Homemade cooling devices – A Thermocol box (small size) filled with cotton or tissue paper, can also be used in place of commercial cooling devices.
Step 6: Store cryogenic vials in liquid nitrogen
◊ Transfer all frozen cryogenic vials to a liquid nitrogen container the next day. Alternatively, frozen cryogenic vials can also be stored in a -150°C freezer.
◊ Cryogenic vials can be stored in the gas phase or liquid phase of liquid nitrogen.
Tip
A frozen vial can be revived after 2 weeks to ensure that frozen stocks are viable and are free of any contamination.
Precautions
1. Biosafety level 2 cell lines should be stored in the gas phase of liquid nitrogen.
2. You must maintain the proper records of the location of frozen cell lines.
3. Wear protective equipment when handling liquid nitrogen. Remember that cryogenic vials may explode if they are stored in a liquid phase of liquid nitrogen.
1 thought on “Protocol: Cryopreservation of Suspension Cell Culture”