Category: Lab Notes: Laboratory Calculations
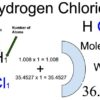
Hydrogen Chloride (HCl) Molecular Weight Calculation
![]()
Hydrogen chloride (HCl) is an inorganic compound of two elements: hydrogen and chlorine. The hydrogen chloride molecular weight is the sum of hydrogen and chlorine atomic weights.
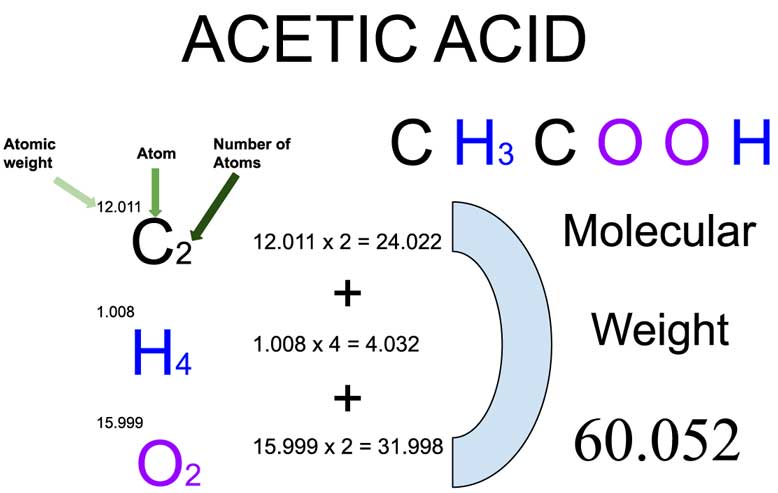
Acetic Acid (CH3COOH) Molecular Weight Calculation
![]()
Acetic acid (CH3COOH) is an organic compound of three elements: Carbon, Hydrogen, and Oxygen. The molecular weight of Ethanolamine is 60.052 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of all its elements.
Calculator: Molar Concentration Conversion
![]()
molarmillimolarmicromolarnanomolarfemtomolarattomolarzeptomolaryoctomolar = molarmillimolarmicromolarnanomolarfemtomolarattomolarzeptomolaryoctomolar molar (M) 101 1 M millimolar (mM) 103 1000 mM micromolar (μM) 106 1000 000 μM nanomolar…
Calculator: Celsius – Kelvin conversion
![]()
FORMULA K = C + 273.15 C = K – 273.15 C = Celsius K = Kelvin CALCULATOR Enter the…
TBE (Tris-Borate-EDTA) Electrophoresis Buffer Calculator
![]()
The TBE (Tris-Borate-EDTA) electrophoresis buffer contains Tris base, Boric acid, EDTA and has pH 8.3. Usually the stock TBE electrophoresis…
Calculator: Converting Percentage by Mass to Molarity
![]()
Please fill the values in the respective box and click the button to calculate the molarity of the solution. Substance…
Molarity of Commercially Supplied Concentrated Acids and Bases
![]()
Acids / bases Molecular formula Molecular weight Strength of Concentrated reagent as supplied commercially Specific gravity (g/ml) at 25 °C…
Molarity of Glacial Acetic Acid (99.7%, w/w, CH3COOH)
![]()
Molarity of Glacial Acetic Acid
Molarity of 56.6% Ammonium Hydroxide (28% Aqueous Solution of Ammonia) [NH4OH (NH3 + H2O)]
![]()
Ammonium hydroxide (NH4OH) is a clear colorless liquid. A 56.6% (w/w) concentrated ammonium hydroxide can be obtained from different suppliers….
Molarity of 70% (w/w) Nitric Acid (HNO3)
![]()
Nitric acid is a clear colorless liquid. A 70% (w/w) concentrated Nitric acid can be obtained from different suppliers. 70%…
Molarity of 95% (w/w) formic acid (HCOOH)
![]()
♦ Formic acid is supplied as a clear colorless liquid. A 95% (w/w) concentrated formic acid can be obtained from…
Molarity of 85% (w/w) Phosphoric acid (H3PO4)
![]()
Phosphoric acid (H3PO4) is a clear colorless liquid. A 85% (w/w) concentrated Phosphoric acid can be obtained from different suppliers….
Molarity of 50% (w/w) Sodium Hydroxide (NaOH)
![]()
A 50% (w/w) concentrated Sodium hydroxide solution is a clear colorless liquid. It is an aqueous solution of Sodium hydroxide (NaOH). The 100 g of 50% Sodium hydroxide solution contains 50 g of NaOH. To calculate the molarity, one must first calculate how much Sodium hydroxide is present in 1 L of 50% Sodium hydroxide solution. Once we know the amount of Sodium hydroxide present in 1 L solution, we can calculate the molarity of the solution by dividing the NaOH amount by the molecular weight.
Molarity of 95% (w/w) Sulfuric acid (H2SO4)
![]()
Sulfuric acid (H2SO4) is a clear colorless liquid. A 95% (w/w) concentrated Sulfuric acid can be obtained from different suppliers….
Molarity of 45% (w/w) Potassium Hydroxide (KOH)
![]()
OVERVIEW A 45% (w/w) concentrated Potassium hydroxide solution is a clear colorless liquid that can be purchased from several commercial…